Ambrisentan (Letairis®)
|
Indications: Ambrisentan is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise ability and delay clinical worsening.
Mechanism: Ambrisentan is a selective antagonist of the endothelin-A (ETA) receptor, binding with high affinity to ETA receptors versus endothelin-B (ETB) rec eptors. The primary actions of ETA are vasoconstriction and cell proliferation, while the predominant actions of ETB are vasodilation, antiproliferation, and endothelin-1 (ET-1) clearance.
Dosing: Initiate treatment at 5 mg once daily with or without food, and consider increasing the dose to
10 mg once daily if 5 mg is tolerated.
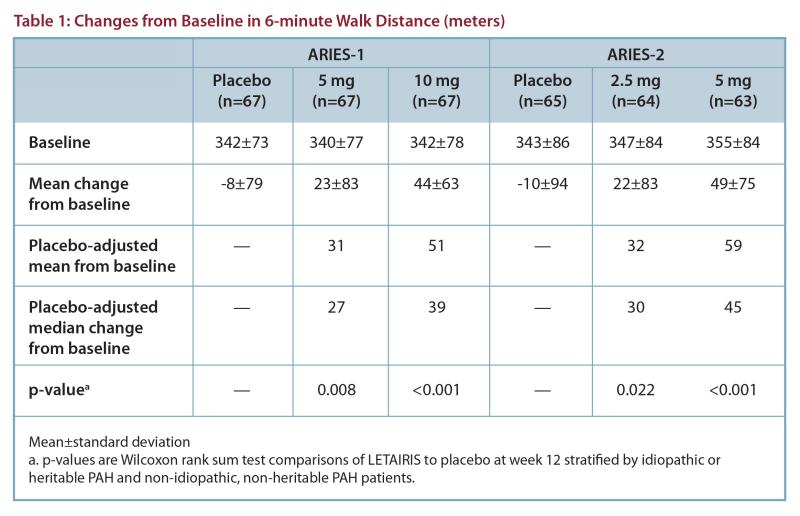
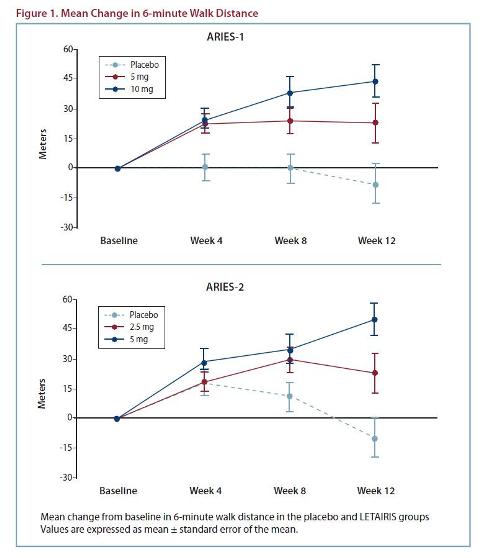
Efficacy: Two 12-week, randomized, double-blind, placebo-controlled, multicenter studies were conducted in 393 patients with PAH (WHO Group 1). ARIES-1 compared once-daily doses of 5 mg and 10 mg ambrisentan to placebo, while ARIES-2 compared once-daily doses of 2.5 mg and 5 mg ambrisentan to placebo, on top of standard therapy. Results of the 6-minute walk distance at 12 weeks for the ARIES-1
and ARIES-2 studies are shown in Table 1 and Figure 1.
|
|
|
|
|
|
|
|
|
In both studies, treatment with ambrisentan resulted in a significant improvement in 6-minute walk distance for each dose of ambrisentan, and the improvements increased with dose. An increase in 6-minute walk distance was observed after 4 weeks of treatment with ambrisentan, with a dose-response observed after
12 weeks of treatment.
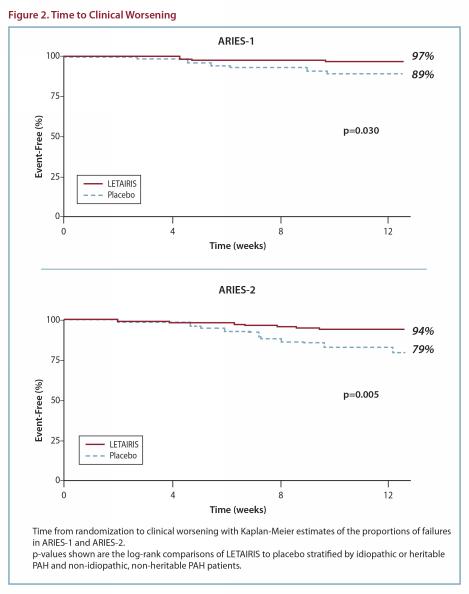
The clinical worsening events during the 12-week treatment period of the ambrisentan clinical trials are shown in Figure 2.
|
|
|
|
|
|
There was a significant delay in the time to clinical worsening for patients receiving ambrisentan compared to placebo. Results in subgroups such as the elderly were also favorable.
Adverse Events: Most common adverse reactions (>3% compared to placebo) are peripheral edema, nasal congestion, sinusitis, and flushing.
Warnings:
- Fluid retention may require intervention.
- If patients develop acute pulmonary edema during initiation of therapy, consider underlying pulmonary veno-occlusive disease and discontinue treatment if necessary.
- Decreases in sperm count have been observed in patients taking endothelin receptor antagonists.
- Decreases in hemoglobin have been observed within the first few weeks; measure hemoglobin at
- initiation, at 1 month, and periodically thereafter.
Contraindications:
- Pregnancy -Do not administer ambrisentan to a pregnant woman because it may cause fetal harm. Exclude pregnancy before the start of treatment.
- Patients with Idiopathic Pulmonary Fibrosis
Metabolism/Drug interactions: Ambrisentan is metabolized by CYP3A, CYP2C19, and other pathways.
- Ambrisentan does not inhibit or induce drug metabolizing enzymes at clinically relevant concentrations.
- Multiple doses of ambrisentan and cyclosporine administered together increases ambrisentan exposure; limit the dose of ambrisentan to 5 mg once daily when administered with cyclosporine.
LEAP Access Program: Because of the risk of birth defects, LETAIRIS is available only through a restricted program called the LETAIRIS Education and Access Program (LEAP).
LEAP Patient Enrollment Form: http://www.letairis.com/downloads/Patient/Enrollment/Guide_LET11585.pdf
LEAP Prescriber Enrollment Form: http://www.letairisrems.com/downloads/Prescriber/Enrollment/Materials_ABS11628_ABS11587.pdf
Full US Prescribing Information can be found here: http://www.gilead.com/~/media/Files/pdfs/medicines/cardiovascular/letairis/letairis_pi.pdf
|